Cocoon Septal Occluder

Flexibility
disc level
Optimal sealing of defects
Designed to minimize nickel leaching
Platinum nanocoating
Softness to protect
Conformability
disc level
Flexibility at the disc level
Cocoon Septal Occluder requires 40.3% less force compared to competitor for displacement at 15 mm indicating higher flexibility of the discs
Displacement at 15 mm
Softness to Protect
Cocoon Septal Occluder requires 30.4% less force compared to competitor for displacement at 8 mm indicating higher softness of the discs
Displacement at 8 mm
Conformability at the discs
Right disc

Cocoon Septal Occluder:
Better conformability of the right atrial disc

Competitor:
Good conformability of the right atrial disc
Cocoon Septal Occluder is highly conformable
Left disc

Cocoon Septal Occluder:
eft atrial disc shows no gap with Cocoon
Septal Occluder

Competitor:
Gap is evident, chances of edge penetration are higher with the Competitor
Cocoon Septal Occluder is highly conformable
Designed to minimize nickel leaching Platinum nanocoating
- Ultrathin layer of Platinum atoms using nanofusion technology is coated on the Nitinol wire by a process called plasma deposition
- Platinum nanocoating makes the Cocoon Occluders inert, biocompatible, non-corrosive and non-allergic and also enhances radiopacity

Raw nitinol

Electropolished

Platinum nanocoating
Platinum nanocoating makes the Cocoon Septal Occluder
Optimal Sealing of Defects
The waist of Cocoon Septal Occluder has adequate sealing strength at the level of the discs: Up to 100% immediate closure rates1, 2

Device Description
- Self-expanding, self-centering, double disc device with unique Platinum nanocoating
- Discs are filled with polypropylene fabric which assists thrombogenicity
- Ability to recapture and redeploy*
- 0% erosion up to 43 months of follow-up in more than 4000 patients3
- 95% to 100% device success rate from early European experience2,4
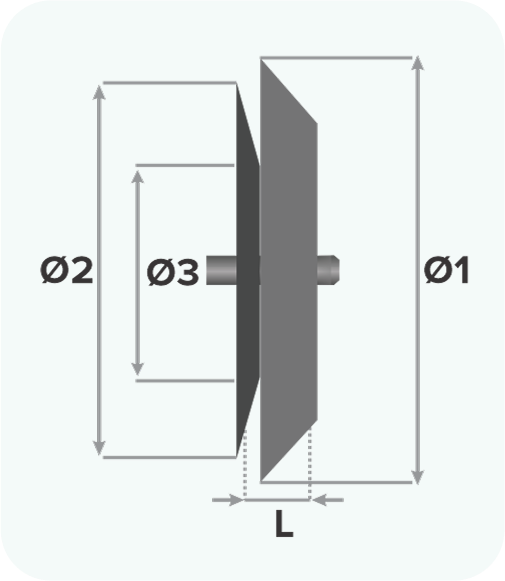
Design
- Left atrial disc diameter (Ø1)
- Device size (Ø3)
- Right atrial disc diameter (Ø2)
- Waist length (L)
Technical Specifications
| Product Code | Device Size (Ø3) (mm) | Left Atrial Disc Diameter (Ø1) (mm) | Waist Length (L) (mm) | Right Atrial Disc Diameter (Ø2) (mm) | Sheath (F) |
|---|---|---|---|---|---|
| COA08 | 8 | 20 | 3 | 18 | 6–7 |
| COA10 | 10 | 22 | 3 | 20 | 6–7 |
| COA12 | 12 | 26 | 4 | 22 | 8–9 |
| COA14 | 14 | 28 | 4 | 24 | 8–9 |
| COA16 | 16 | 30 | 4 | 26 | 8–9 |
| COA18 | 18 | 32 | 4 | 28 | 10–12 |
| COA20 | 20 | 34 | 4 | 30 | 10–12 |
| COA22 | 22 | 36 | 4 | 32 | 10–12 |
| COA24 | 24 | 38 | 4 | 34 | 10–12 |
| COA26 | 26 | 40 | 4 | 36 | 10–12 |
| COA28 | 28 | 42 | 4 | 38 | 10–12 |
| COA30 | 30 | 44 | 4 | 40 | 12–14 |
| COA32 | 32 | 46 | 4 | 42 | 12–14 |
| COA34 | 34 | 50 | 4 | 44 | 12–14 |
| COA36 | 36 | 52 | 4 | 46 | 12–14 |
| COA38 | 38 | 54 | 4 | 48 | 12–14 |
| COA40 | 40 | 56 | 4 | 50 | 12–14 |
| COA42 | 42 | 58 | 4 | 52 | 14 |
Usable lengths of compatible accessories (cm)
Usable Length (cm)
| 6F | 7F | 8F | 9F | 10F | 12F | 14F | |
|---|---|---|---|---|---|---|---|
| Sheath | 60 | 60 | 78 | 78 | 78 | 78 | 78 |
| Dilator | 66 | 66 | 84 | 84 | 84 | 84 | 84 |
| Loader | 9 | 9 | 9 | 11 | 11 | 13 | 13 |
Delivery Cable
| Description | Profile (inch) | Length (cm) | Catalog Number |
|---|---|---|---|
| 6F Delivery Cable | 0.077 | 117 | CDC6F |
Preclinical Studies
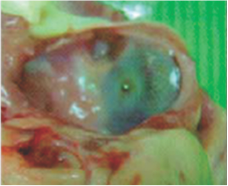
Neoendothelialization on the left atrial surface of the device at 36 days after implantation
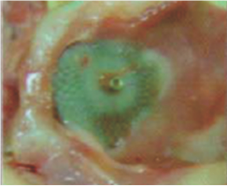
Neoendothelialization on the right atrial surface of the device at 36 days after implantation
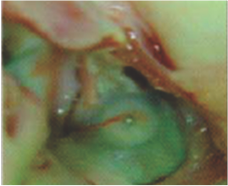
Neoendothelialization on the left atrial surface of the device at 42 days after implantation
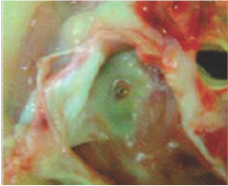
Neoendothelialization on the right atrial surface of the device at 42 days after implantation
Caution
This product is intended for use by or under the direction of a physician. Prior to use, refer to the “Instructions for use” supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.