Cocoon Duct Occluder

Platinum nanocoating technology
Nitinol wire used in the Cocoon Duct Occluder is coated with Platinum atoms using nano fusion technology by plasma deposition

Raw nitinol

Electropolished

Platinum nanocoating
Platinum nanocoating makes the Cocoon Septal Occluder
Key highlights
- Hat-shaped design ensures secured implantation at the aorta and minimizes risk of migration into pulmonary artery
- Unique Platinum nanocoating
- Device is filled with polypropylene fabric to assist thrombogenicity
- Extended retention portion provides proper positioning of the device in the ductus arteriosus
- 100% occlusion rate at one-month follow-up1
- Ability to recapture and redeploy*
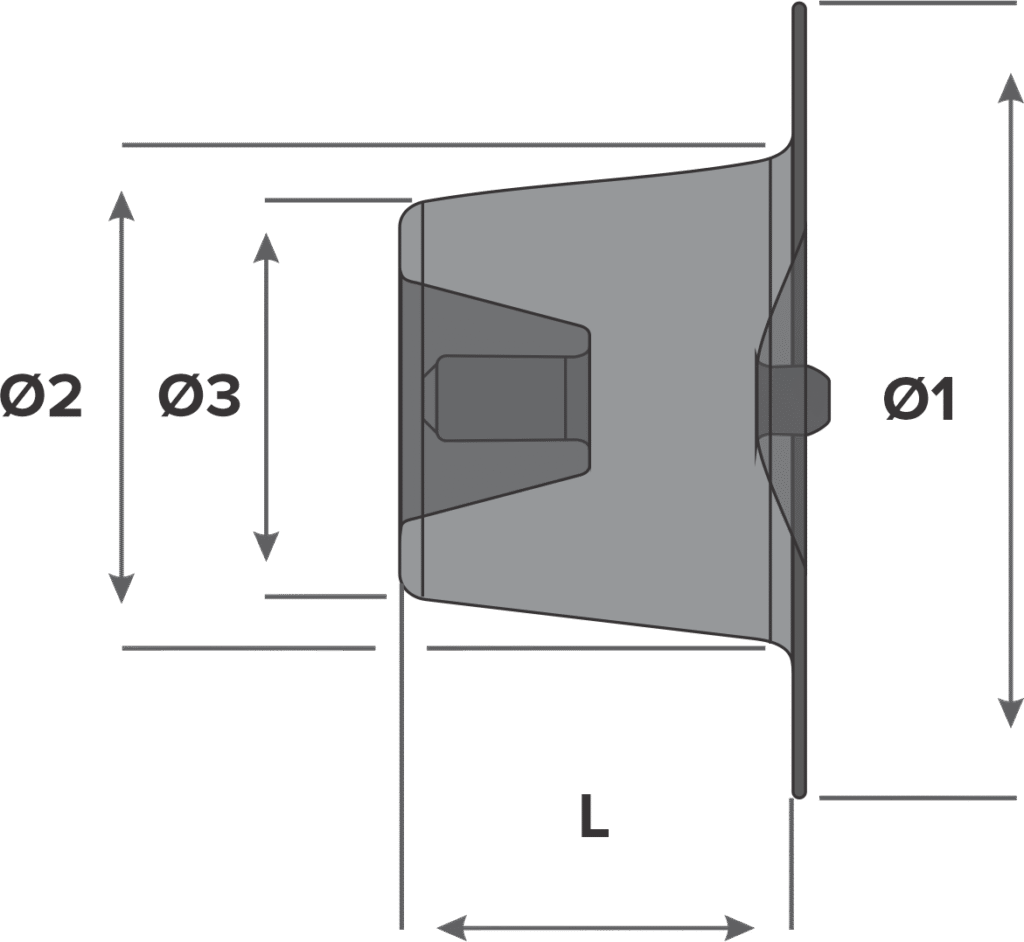
Design
- Retention skirt diameter (Ø1)
- Device diameter at descending aorta (Ø2)
- Device diameter at pulmonary artery (Ø3)
- Device length (L)
Technical Specifications
| Product Code | Retention skirt diameter (Ø1) (mm) | Device diameter at descending aorta (Ø2) (mm) | Device diameter at pulmonary artery (Ø3) (mm) | Device length (L) (mm) | CompatibleSheath Size (F) |
|---|---|---|---|---|---|
| COP0406 | 10 | 6 | 4 | 7 | 6-7 |
| COP0608 | 12 | 8 | 6 | 7 | 6-7 |
| COP0810 | 16 | 10 | 8 | 8 | 7-8 |
| COP1012 | 18 | 12 | 10 | 8 | 7-8 |
| COP1214 | 20 | 14 | 12 | 8 | 8-9 |
| COP1416 | 22 | 16 | 14 | 8 | 8-9 |
| COP1618 | 24 | 18 | 16 | 8 | 9 |
| COP1820 | 26 | 20 | 18 | 8 | 9 |
Usable lengths of compatible accessories (cm)
Usable Length (cm)
| 6F | 7F | 8F | 9F | |
|---|---|---|---|---|
| Sheath | 83 | 83 | 83 | 83 |
| Dilator | 89 | 89 | 89 | 89 |
| Loader | 10 | 10 | 10 | 12 |
Delivery Cable
| Description | Profile (inch) | Length (cm) | Catalog Number |
|---|---|---|---|
| 5F Delivery Cable | 0.043 | 125 | CDC5F |
| 6F Delivery Cable | 0.077 | 117 | CDC6F |
Preclinical Studies
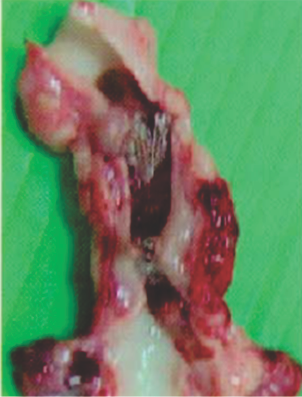
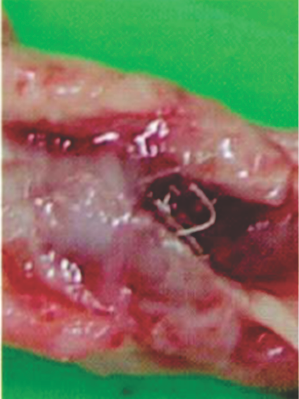
Seven days after implantation in a pig PDA shows a well-encapsulated device
Caution
This product is intended for use by or under the direction of a physician. Prior to use, refer to the “Instructions for use” supplied with these devices for indications, contraindications, side effects, suggested procedure warnings and precautions. As part of our continuous product development policy, we reserve the right to change product specifications without prior notification. Information contained herein is for distribution outside the USA & Japan.